Cefmenoksim
Cefmenoksim je organsko jedinjenje, koje sadrži 16 atoma ugljenika i ima molekulsku masu od 511,558 Da.[1][2][3][4][5][6]
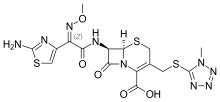 | |
| Klinički podaci | |
|---|---|
| Prodajno ime | Bestcall, Cefmax, Tacef |
| Drugs.com | Monografija |
| Način primene | Intramaskularno |
| Farmakokinetički podaci | |
| Poluvreme eliminacije | 1 h |
| Identifikatori | |
| CAS broj | 65085-01-0 |
| ATC kod | J01DD05 (WHO) |
| PubChem | CID 9570757 |
| DrugBank | DB00267 |
| ChemSpider | 7845223 |
| ChEBI | CHEBI:55490 |
| ChEMBL | CHEMBL1201224 |
| Hemijski podaci | |
| Formula | C16H17N9O5S3 |
| Molarna masa | 511,558 |
| |
| |
Osobine уреди
| Osobina | Vrednost |
|---|---|
| Broj akceptora vodonika | 13 |
| Broj donora vodonika | 3 |
| Broj rotacionih veza | 8 |
| Particioni koeficijent[7] (ALogP) | -0,7 |
| Rastvorljivost[8] (logS, log(mol/L)) | -3,8 |
| Polarna površina[9] (PSA, Å2) | 269,6 |
Reference уреди
- ^ Yokota N, Koguchi M, Suzuki Y, Fukayama S, Ishihara R, Deguchi K, Oda S, Tanaka S, Nakane Y, Fukumoto T: [Antibacterial activities of cefmenoxime against recent fresh clinical isolates from patients in sinusitis] Jpn J Antibiot. 1995 May;48(5):602-9. PMID 7637194
- ^ Paladino JA, Fell RE: Pharmacoeconomic analysis of cefmenoxime dual individualization in the treatment of nosocomial pneumonia. Ann Pharmacother. 1994 Mar;28(3):384-9. PMID 8193431
- ^ Duncker GI, Reich U, Krausse R: Cefmenoxime in corneal organ culture. Ophthalmologica. 1994;208(5):262-6. PMID 7816419
- ^ Tsuchiya K, Kondo M, Kida M, Nakao M, Iwahi T, Nishi T, Noji Y, Takeuchi M, Nozaki Y: Cefmenoxime (SCE-1365), a novel broad-spectrum cephalosporin: in vitro and in vivo antibacterial activities. Antimicrob Agents Chemother. 1981 Jan;19(1):56-65. PMID 6941742
- ^ Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS (2011). „DrugBank 3.0: a comprehensive resource for omics research on drugs”. Nucleic Acids Res. 39 (Database issue): D1035—41. PMC 3013709 . PMID 21059682. doi:10.1093/nar/gkq1126.
- ^ David S. Wishart; Craig Knox; An Chi Guo; Dean Cheng; Savita Shrivastava; Dan Tzur; Bijaya Gautam; Murtaza Hassanali (2008). „DrugBank: a knowledgebase for drugs, drug actions and drug targets”. Nucleic acids research. 36 (Database issue): D901—6. PMC 2238889 . PMID 18048412. doi:10.1093/nar/gkm958.
- ^ Ghose, A.K.; Viswanadhan V.N. & Wendoloski, J.J. (1998). „Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragment Methods: An Analysis of AlogP and CLogP Methods”. J. Phys. Chem. A. 102: 3762—3772. doi:10.1021/jp980230o.
- ^ Tetko IV, Tanchuk VY, Kasheva TN, Villa AE (2001). „Estimation of Aqueous Solubility of Chemical Compounds Using E-State Indices”. Chem Inf. Comput. Sci. 41: 1488—1493. PMID 11749573. doi:10.1021/ci000392t.
- ^ Ertl P.; Rohde B.; Selzer P. (2000). „Fast calculation of molecular polar surface area as a sum of fragment based contributions and its application to the prediction of drug transport properties”. J. Med. Chem. 43: 3714—3717. PMID 11020286. doi:10.1021/jm000942e.
Literatura уреди
- Hardman JG, Limbird LE, Gilman AG (2001). Goodman & Gilman's The Pharmacological Basis of Therapeutics (10. изд.). New York: McGraw-Hill. ISBN 0071354697. doi:10.1036/0071422803.
- Thomas L. Lemke; David A. Williams, ур. (2007). Foye's Principles of Medicinal Chemistry (6. изд.). Baltimore: Lippincott Willams & Wilkins. ISBN 0781768799.
Spoljašnje veze уреди
| Molimo Vas, obratite pažnju na važno upozorenje u vezi sa temama iz oblasti medicine (zdravlja). |