Gadobutrol
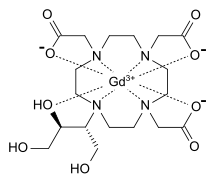
Gadobutrol je organsko jedinjenje, koje sadrži 18 atoma ugljenika i ima molekulsku masu od 604,710 Da.[1][2][3][4][5]
 | |
| Klinički podaci | |
|---|---|
| Prodajno ime | Gadavist, Gadovist |
| Drugs.com | Monografija |
| Način primene | Intravenozno |
| Farmakokinetički podaci | |
| Poluvreme eliminacije | 1,8 h |
| Izlučivanje | Renalno |
| Identifikatori | |
| CAS broj | 138071-82-6 |
| ATC kod | V08CA09 (WHO) |
| PubChem | CID 72057 |
| DrugBank | DB06703 |
| ChEBI | CHEBI:68841 |
| ChEMBL | CHEMBL1628503 |
| Hemijski podaci | |
| Formula | C18H31N4O9 |
| Molarna masa | 604,710 |
| |
| |
Osobine уреди
| Osobina | Vrednost |
|---|---|
| Broj akceptora vodonika | 13 |
| Broj donora vodonika | 3 |
| Broj rotacionih veza | 10 |
| Particioni koeficijent[6] (ALogP) | -7,0 |
| Rastvorljivost[7] (logS, log(mol/L)) | 3,0 |
| Polarna površina[8] (PSA, Å2) | 244,3 |
Reference уреди
- ^ Scott, Lesley J. (2013). „Gadobutrol: A Review of Its Use for Contrast-Enhanced Magnetic Resonance Imaging in Adults and Children”. Clinical Drug Investigation. 33 (4): 303—314. PMID 23435930. S2CID 207483597. doi:10.1007/s40261-013-0066-0.
- ^ Wack, Christiane; Steger-Hartmann, Thomas; Mylecraine, Louis; Hofmeister, Rainer (2012). „Toxicological Safety Evaluation of Gadobutrol”. Investigative Radiology. 47 (11): 611—623. PMID 23011188. S2CID 10146613. doi:10.1097/RLI.0b013e318263f128.
- ^ Künnemeyer, Jens; Terborg, Lydia; Nowak, Sascha; Scheffer, Andy; Telgmann, Lena; Tokmak, Faruk; Günsel, Andreas; Wiesmüller, Gerhard; Reichelt, Stephan; Karst, Uwe (2008). „Speciation Analysis of Gadolinium-Based MRI Contrast Agents in Blood Plasma by Hydrophilic Interaction Chromatography/Electrospray Mass Spectrometry”. Analytical Chemistry. 80 (21): 8163—8170. PMID 18821778. doi:10.1021/ac801264j.
- ^ Knox, C.; Law, V.; Jewison, T.; Liu, P.; Ly, S.; Frolkis, A.; Pon, A.; Banco, K.; Mak, C.; Neveu, V.; Djoumbou, Y.; Eisner, R.; Guo, A. C.; Wishart, D. S. (2011). „DrugBank 3.0: A comprehensive resource for 'omics' research on drugs”. Nucleic Acids Research. 39 (Database issue): D1035—41. PMC 3013709 . PMID 21059682. doi:10.1093/nar/gkq1126.
- ^ Wishart, D. S.; Knox, C.; Guo, A. C.; Cheng, D.; Shrivastava, S.; Tzur, D.; Gautam, B.; Hassanali, M. (2008). „DrugBank: A knowledgebase for drugs, drug actions and drug targets”. Nucleic Acids Research. 36 (Database issue): D901—6. PMC 2238889 . PMID 18048412. doi:10.1093/nar/gkm958.
- ^ Ghose, A.K.; Viswanadhan V.N. & Wendoloski, J.J. (1998). „Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragment Methods: An Analysis of AlogP and CLogP Methods”. J. Phys. Chem. A. 102: 3762—3772. doi:10.1021/jp980230o.
- ^ Tetko, I. V.; Tanchuk, V. Y.; Kasheva, T. N.; Villa, A. E. (2001). „Estimation of aqueous solubility of chemical compounds using E-state indices”. Journal of Chemical Information and Computer Sciences. 41 (6): 1488—1493. PMID 11749573. doi:10.1021/ci000392t.
- ^ Ertl, P.; Rohde, B.; Selzer, P. (2000). „Fast calculation of molecular polar surface area as a sum of fragment-based contributions and its application to the prediction of drug transport properties”. Journal of Medicinal Chemistry. 43 (20): 3714—3717. PMID 11020286. doi:10.1021/jm000942e.
Literatura уреди
- Hardman JG, Limbird LE, Gilman AG (2001). Goodman & Gilman's The Pharmacological Basis of Therapeutics (10. изд.). New York: McGraw-Hill. ISBN 0071354697. doi:10.1036/0071422803.
- Thomas L. Lemke; David A. Williams, ур. (2007). Foye's Principles of Medicinal Chemistry (6. изд.). Baltimore: Lippincott Willams & Wilkins. ISBN 0781768799.
Spoljašnje veze уреди
| Molimo Vas, obratite pažnju na važno upozorenje u vezi sa temama iz oblasti medicine (zdravlja). |