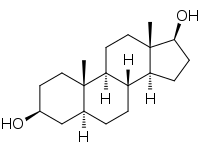
3β-Androstandiol
3β-Androstandiol je androstandiol[3] koji je izveden iz DHT u reakciji katalizovanoj enzimom 3-β-HSD
| |
| Nazivi | |
|---|---|
| IUPAC naziv
(3S,5S,8R,9S,10S,13S,14S,17S)-10,13-Dimetil-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradekahidro-1H-ciklopenta[a]fenantren-3,17-diol
| |
| Drugi nazivi
3beta,17beta-androstandiol
| |
| Identifikacija | |
3D model (Jmol)
|
|
| ChemSpider | |
| ECHA InfoCard | 100.008.487 |
| |
| Svojstva | |
| C19H32O2 | |
| Molarna masa | 292,46 g/mol |
Ukoliko nije drugačije napomenuto, podaci se odnose na standardno stanje materijala (na 25 °C [77 °F], 100 kPa). | |
| Reference infokutije | |
Reference
уреди- ^ Li Q, Cheng T, Wang Y, Bryant SH (2010). „PubChem as a public resource for drug discovery.”. Drug Discov Today. 15 (23-24): 1052—7. PMID 20970519. doi:10.1016/j.drudis.2010.10.003.
- ^ Evan E. Bolton; Yanli Wang; Paul A. Thiessen; Stephen H. Bryant (2008). „Chapter 12 PubChem: Integrated Platform of Small Molecules and Biological Activities”. Annual Reports in Computational Chemistry. 4: 217—241. doi:10.1016/S1574-1400(08)00012-1.
- ^ Chambon C; Bennat D; Delolme F; et al. (2001). „Photoaffinity labeling of human sex hormone-binding globulin using 17alpha-alkylamine derivatives of 3beta-androstanediol substituted with azidonitrophenylamido, azidonitrophenylamino, or trifluoroazidonitrophenylamino chromophores. Localization of Trp-84 in the vicinity of the steroid-binding site”. Biochemistry. 40 (50): 15424—35. PMID 11735427. doi:10.1021/bi011504s.