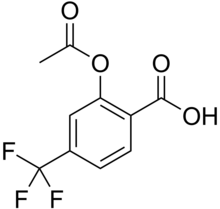
Triflusal
Triflusal je antiagregacijski lek koji se koristi za sekondarnu prevenciju aterombotiskog srčanog udara. Klinička ispitivanja su pokazala da je triflusal jednako efektivan i da ima bolji bezbednosni profil od acetilsalicilne kiseline plus dipridamol i klopidogrel.[1][2][3][4][5][6][7][8][9][10][11][12]
 | |
| Klinički podaci | |
|---|---|
| Drugs.com | Monografija |
| Način primene | Oralno |
| Farmakokinetički podaci | |
| Poluvreme eliminacije | 0,5 +/- 0,1h |
| Izlučivanje | Renalno |
| Identifikatori | |
| CAS broj | 322-79-2 |
| ATC kod | B01AC18 (WHO) |
| PubChem | CID 9458 |
| DrugBank | DB08814 |
| ChEMBL | CHEMBL1332032 |
| Hemijski podaci | |
| Formula | C10H7F3O4 |
| Molarna masa | 248,155 g/mol |
| |
| |
Reference
уреди- ^ Anninos H, Andrikopoulos G, Pastromas S, Sakellariou D, Theodorakis G, Vardas P: Triflusal: an old drug in modern antiplatelet therapy. Review of its action, use, safety and effectiveness. Hellenic J Cardiol. 2009 May-Jun;50(3):199-207. PMID 19465361
- ^ Izquierdo I, Borja J, Rovira S, Pelagio P, Torres F, Cebrecos J, Garcia-Rafanell J: Comparative bioavailability study of triflusal oral solution vs. triflusal capsules in healthy subjects. A single, randomized, two-way cross-over, open-label phase I study. Arzneimittelforschung. 2010;60(1):36-41. PMID 20184225
- ^ Duran X, Sanchez S, Vilahur G, Badimon L: Protective effects of triflusal on secondary thrombus growth and vascular cyclooxygenase-2. J Thromb Haemost. 2008 Aug;6(8):1385-92. Epub 2008 May 22. PMID 18503633
- ^ Quetglas EG, Campanero MA, Sadaba B, Escolar M, Azanza JR: Bioequivalence of two oral formulations of triflusal capsules in healthy volunteers. Arzneimittelforschung. 2008;58(6):283-7. PMID 18677970
- ^ Costa J, Ferro JM, Matias-Guiu J, Alvarez-Sabin J, Torres F: Triflusal for Preventing Serious Vascular Events in People at High Risk. Stroke. 2006 Jun 22. PMID 16794202
- ^ Gonzalez-Correa JA, De La Cruz JP: Triflusal: an antiplatelet drug with a neuroprotective effect? Cardiovasc Drug Rev. 2006 Spring;24(1):11-24. PMID 16939630
- ^ Fraj J, Valero A, Vives R, Perez I, Borja J, Izquierdo I, Picado C: Safety of triflusal (antiplatelet drug) in patients with aspirin-exacerbated respiratory diseases. Allergy. 2008 Jan;63(1):112-5. PMID 18053020
- ^ Sanchez-Machin I, Garcia Robaina JC, Torre Morin F: Widespread eczema from triflusal confirmed by patch testing. Contact Dermatitis. 2004 Apr;50(4):257. PMID 15186390
- ^ Matias-Guiu J, Ferro JM, Alvarez-Sabin J, Torres F, Jimenez MD, Lago A, Melo T: Comparison of triflusal and aspirin for prevention of vascular events in patients after cerebral infarction: the TACIP Study: a randomized, double-blind, multicenter trial. Stroke. 2003 Apr;34(4):840-8. Epub 2003 Mar 20. PMID 12649515
- ^ Culebras A, Rotta-Escalante R, Vila J, Dominguez R, Abiusi G, Famulari A, Rey R, Bauso-Tosselli L, Gori H, Ferrari J, Reich E: Triflusal vs aspirin for prevention of cerebral infarction: a randomized stroke study. Neurology. 2004 Apr 13;62(7):1073-80. PMID 15079004
- ^ Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS (2011). „DrugBank 3.0: a comprehensive resource for omics research on drugs”. Nucleic Acids Res. 39 (Database issue): D1035—41. PMC 3013709 . PMID 21059682. doi:10.1093/nar/gkq1126.
- ^ David S. Wishart; Craig Knox; An Chi Guo; Dean Cheng; Savita Shrivastava; Dan Tzur; Bijaya Gautam; Murtaza Hassanali (2008). „DrugBank: a knowledgebase for drugs, drug actions and drug targets”. Nucleic acids research. 36 (Database issue): D901—6. PMC 2238889 . PMID 18048412. doi:10.1093/nar/gkm958.
Literatura
уреди- Hardman JG, Limbird LE, Gilman AG (2001). Goodman & Gilman's The Pharmacological Basis of Therapeutics (10. изд.). New York: McGraw-Hill. ISBN 0071354697. doi:10.1036/0071422803.
- Thomas L. Lemke; David A. Williams, ур. (2007). Foye's Principles of Medicinal Chemistry (6. изд.). Baltimore: Lippincott Willams & Wilkins. ISBN 0781768799.
Spoljašnje veze
уреди
| Molimo Vas, obratite pažnju na važno upozorenje u vezi sa temama iz oblasti medicine (zdravlja). |