Fenobarbital
Fenobarbital je derivat barbituratne kiseline, koji deluje kao neselektivni depresant centralnog nervnog sistema. On promoviše vezivanje za inhibitorni tip receptora gama-aminobuterine kiseline, i moduliše protok hlorida kroz receptorske kanale. On snažno inhibira glutamatom indukovane depolarizacije.[1][2][3][4][5][6][7]
 | |
 | |
| IUPAC ime | |
|---|---|
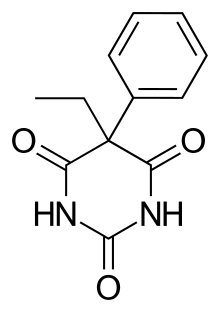
5-etil-5-phenil-1,3-diazinan-2,4,6-trion | |
| Klinički podaci | |
| Prodajno ime | Adonal, Aephenal, Agrypnal, Amylofene |
| Drugs.com | Monografija |
| Način primene | Oralno; Intramaskularno; Oralno; |
| Farmakokinetički podaci | |
| Poluvreme eliminacije | 53 do 118 sati |
| Identifikatori | |
| CAS broj | 50-06-6 |
| ATC kod | N03AA01 (WHO), N03AA02 |
| PubChem | CID 4763 |
| IUPHAR/BPS | 2804 |
| DrugBank | DB01174 |
| ChemSpider | 4599 |
| KEGG | C07434 |
| ChEMBL | CHEMBL8069 |
| Hemijski podaci | |
| Formula | C12H12N2O3 |
| Molarna masa | 232.2353 |
| |
| |
| Fizički podaci | |
| Tačka topljenja | 174 °C (345 °F) |
Reference уреди
- ^ Kwan P, Brodie MJ: Phenobarbital for the treatment of epilepsy in the 21st century: a critical review. Epilepsia. 2004 Sep;45(9):1141-9. PMID 15329080
- ^ Taylor S, Tudur Smith C, Williamson PR, Marson AG: Phenobarbitone versus phenytoin monotherapy for partial onset seizures and generalized onset tonic-clonic seizures. Cochrane Database Syst Rev. 2001;(4):CD002217. PMID 11687150
- ^ Tudur Smith C, Marson AG, Williamson PR: Carbamazepine versus phenobarbitone monotherapy for epilepsy. Cochrane Database Syst Rev. 2003;(1):CD001904. PMID 12535420
- ^ Kalviainen R, Eriksson K, Parviainen I: Refractory generalised convulsive status epilepticus : a guide to treatment. CNS Drugs. 2005;19(9):759-68. PMID 16142991
- ^ Booth D, Evans DJ: Anticonvulsants for neonates with seizures. Cochrane Database Syst Rev. 2004 Oct 18;(4):CD004218. PMID 15495087
- ^ Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS (2011). „DrugBank 3.0: a comprehensive resource for omics research on drugs”. Nucleic Acids Res. 39 (Database issue): D1035—41. PMID 21059682.
- ^ Wishart DS, Knox C, Guo AC, Cheng D, Shrivastava S, Tzur D, Gautam B, Hassanali M (Nucleic Acids Res) (2008). „DrugBank: a knowledgebase for drugs, drug actions and drug targets”. 36 (Database issue): D901—6. PMID 18048412.
Spoljašnje veze уреди
| Molimo Vas, obratite pažnju na važno upozorenje u vezi sa temama iz oblasti medicine (zdravlja). |