LY-379,268
LY-379,268 je lek koji se koristi u neurološkim istraživanjima. On deluje kao potentan i selektivan agonist za grupu II metabotropnih glutamatnih receptora (mGluR2/3).
 | |
| IUPAC ime | |
|---|---|
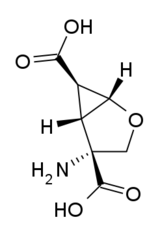
(1S,2R,5R,6R)-2-amino-4-oksabiciklo[3.1.0]heksan-2,6-dikarboksilna kiselina | |
| Identifikatori | |
| CAS broj | 191471-52-0 |
| ATC kod | none |
| PubChem | CID 10197984 |
| Hemijski podaci | |
| Formula | C7H9NO5 |
| Molarna masa | 187,150 g/mol |
| |
On je izveden iz starijeg agonista mGluR groupe II eglumegada,[1] i doveo je do razvoja potentnijeg jedinjenja LY-404,039,[2] ali se još uvek koristi u istraživanjima. LY-379,268 ima sedativne, neuroprotektivne, antizavisničke i antikonvulsantske efekte na životinjama,[3][4][5] i blokira dejstvo PCP-a i DOI-a,[6][7][8][9] što je dovelo do ispitivanja LY-379,268 i sličnih jedinjenja kao potencijalnih antipsihotičnih lekova za tretman šizovrenije.[10][11]
Reference
uredi- ^ Monn JA, Valli MJ, Massey SM, Hansen MM, Kress TJ, Wepsiec JP, Harkness AR, Grutsch JL, Wright RA, Johnson BG, Andis SL, Kingston A, Tomlinson R, Lewis R, Griffey KR, Tizzano JP, Schoepp DD (1999). „Synthesis, pharmacological characterization, and molecular modeling of heterobicyclic amino acids related to (+)-2-aminobicyclo[3.1.0] hexane-2,6-dicarboxylic acid (LY354740): identification of two new potent, selective, and systemically active agonists for group II metabotropic glutamate receptors”. Journal of Medicinal Chemistry. 42 (6): 1027—40. PMID 10090786. doi:10.1021/jm980616n.
- ^ Rorick-Kehn LM, Johnson BG, Burkey JL, Wright RA, Calligaro DO, Marek GJ, Nisenbaum ES, Catlow JT, Kingston AE, Giera DD, Herin MF, Monn JA, McKinzie DL, Schoepp DD (2007). „Pharmacological and pharmacokinetic properties of a structurally novel, potent, and selective metabotropic glutamate 2/3 receptor agonist: in vitro characterization of agonist (-)-(1R,4S,5S,6S)-4-amino-2-sulfonylbicyclo[3.1.0]-hexane-4,6-dicarboxylic acid (LY404039)”. The Journal of Pharmacology and Experimental Therapeutics. 321 (1): 308—17. PMID 17204749. doi:10.1124/jpet.106.110809.
- ^ Cai Z, Xiao F, Fratkin JD, Rhodes PG (1999). „Protection of neonatal rat brain from hypoxic-ischemic injury by LY379268, a Group II metabotropic glutamate receptor agonist”. Neuroreport. 10 (18): 3927—31. PMID 10716235. doi:10.1097/00001756-199912160-00037.
- ^ Moldrich RX, Jeffrey M, Talebi A, Beart PM, Chapman AG, Meldrum BS (2001). „Anti-epileptic activity of group II metabotropic glutamate receptor agonists (-)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate (LY379268) and (-)-2-thia-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate (LY389795)”. Neuropharmacology. 41 (1): 8—18. PMID 11445181. doi:10.1016/S0028-3908(01)00044-2.
- ^ Uys JD, LaLumiere RT (2008). „Glutamate: the new frontier in pharmacotherapy for cocaine addiction”. CNS & Neurological Disorders Drug Targets. 7 (5): 482—91. PMID 19128205. doi:10.2174/187152708786927868.
- ^ Cartmell J, Monn JA, Schoepp DD (2000). „Attenuation of specific PCP-evoked behaviors by the potent mGlu2/3 receptor agonist, LY379268 and comparison with the atypical antipsychotic, clozapine”. Psychopharmacology. 148 (4): 423—9. PMID 10928316. doi:10.1007/s002130050072.
- ^ Greenslade RG, Mitchell SN (2004). „Selective action of (-)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate (LY379268), a group II metabotropic glutamate receptor agonist, on basal and phencyclidine-induced dopamine release in the nucleus accumbens shell”. Neuropharmacology. 47 (1): 1—8. PMID 15165829. doi:10.1016/j.neuropharm.2004.02.015.
- ^ Kłodzinska A, Bijak M, Tokarski K, Pilc A (2002). „Group II mGlu receptor agonists inhibit behavioural and electrophysiological effects of DOI in mice”. Pharmacology, Biochemistry, and Behavior. 73 (2): 327—32. PMID 12117586. doi:10.1016/S0091-3057(02)00845-6.
- ^ Molinaro G, Traficante A, Riozzi B, Di Menna L, Curto M, Pallottino S, Nicoletti F, Bruno V, Battaglia G (2009). „Activation of mGlu2/3 metabotropic glutamate receptors negatively regulates the stimulation of inositol phospholipid hydrolysis mediated by 5-hydroxytryptamine2A serotonin receptors in the frontal cortex of living mice”. Molecular Pharmacology. 76 (2): 379—87. PMID 19439499. doi:10.1124/mol.109.056580.
- ^ Carter K, Dickerson J, Schoepp DD, Reilly M, Herring N, Williams J, Sallee FR, Sharp JW, Sharp FR (2004). „The mGlu2/3 receptor agonist LY379268 injected into cortex or thalamus decreases neuronal injury in retrosplenial cortex produced by NMDA receptor antagonist MK-801: possible implications for psychosis”. Neuropharmacology. 47 (8): 1135—45. PMID 15567423. doi:10.1016/j.neuropharm.2004.08.018.
- ^ Imre G (2007). „The preclinical properties of a novel group II metabotropic glutamate receptor agonist LY379268”. CNS Drug Reviews. 13 (4): 444—64. PMID 18078428. doi:10.1111/j.1527-3458.2007.00024.x.