Azacitidin
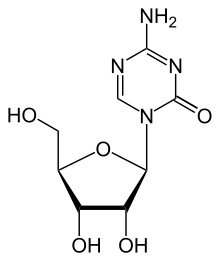
Azacitidin je organsko jedinjenje, koje sadrži 8 atoma ugljenika i ima molekulsku masu od 244,205 Da.[1][2][3][4][5][6][7][8][9][10][11][12][13][14][15]
 | |
| Klinički podaci | |
|---|---|
| Prodajno ime | Ladakamycin, Mylosar, Vidaza |
| Drugs.com | Monografija |
| Način primene | Subkutano |
| Farmakokinetički podaci | |
| Poluvreme eliminacije | 4 h |
| Izlučivanje | Renalno |
| Identifikatori | |
| CAS broj | 320-67-2 |
| ATC kod | None |
| PubChem | CID 9444 |
| DrugBank | DB00928 |
| ChemSpider | 9072 |
| KEGG | C11262 |
| ChEBI | CHEBI:2038 |
| ChEMBL | CHEMBL1489 |
| Hemijski podaci | |
| Formula | C8H12N4O5 |
| Molarna masa | 244,205 |
| |
| |
| Fizički podaci | |
| Tačka topljenja | 229 °C (444 °F) |
Osobine
уреди| Osobina | Vrednost |
|---|---|
| Broj akceptora vodonika | 8 |
| Broj donora vodonika | 4 |
| Broj rotacionih veza | 2 |
| Particioni koeficijent[16] (ALogP) | -1,9 |
| Rastvorljivost[17] (logS, log(mol/L)) | -0,3 |
| Polarna površina[18] (PSA, Å2) | 141,0 |
Reference
уреди- ^ Cihak A: Biological effects of 5-azacytidine in eukaryotes. Oncology. 1974;30(5):405-22. PMID 4142650
- ^ Kaminskas E, Farrell AT, Wang YC, Sridhara R, Pazdur R: FDA drug approval summary: azacitidine (5-azacytidine, Vidaza) for injectable suspension. Oncologist. 2005 Mar;10(3):176-82. PMID 15793220
- ^ Leone G, Voso MT, Teofili L, Lubbert M: Inhibitors of DNA methylation in the treatment of hematological malignancies and MDS. Clin Immunol. 2003 Oct;109(1):89-102. PMID 14585280
- ^ Ghoshal K, Bai S: DNA methyltransferases as targets for cancer therapy. Drugs Today (Barc). 2007 Jun;43(6):395-422. PMID 17612710
- ^ Silverman LR, Demakos EP, Peterson BL, Kornblith AB, Holland JC, Odchimar-Reissig R, Stone RM, Nelson D, Powell BL, DeCastro CM, Ellerton J, Larson RA, Schiffer CA, Holland JF: Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002 May 15;20(10):2429-40. PMID 12011120
- ^ Silverman LR: Targeting hypomethylation of DNA to achieve cellular differentiation in myelodysplastic syndromes (MDS). Oncologist. 2001;6 Suppl 5:8-14. PMID 11700387
- ^ Issa JP, Kantarjian H: Azacitidine. Nat Rev Drug Discov. 2005 May;Suppl:S6-7. PMID 15962522
- ^ O'Dwyer K, Maslak P: Azacitidine and the beginnings of therapeutic epigenetic modulation. Expert Opin Pharmacother. 2008 Aug;9(11):1981-6. PMID 18627335
- ^ Siddiqui MA, Scott LJ: Azacitidine: in myelodysplastic syndromes. Drugs. 2005;65(13):1781-9; discussion 1790-1. PMID 16114977
- ^ Abdulhaq H, Rossetti JM: The role of azacitidine in the treatment of myelodysplastic syndromes. Expert Opin Investig Drugs. 2007 Dec;16(12):1967-75. PMID 18042004
- ^ Keating GM: Azacitidine: a review of its use in higher-risk myelodysplastic syndromes/acute myeloid leukaemia. Drugs. 2009;69(17):2501-18. doi: 10.2165/11202840-000000000-00000. PMID 19911860
- ^ Sullivan M, Hahn K, Kolesar JM: Azacitidine: a novel agent for myelodysplastic syndromes. Am J Health Syst Pharm. 2005 Aug 1;62(15):1567-73. PMID 16030365
- ^ Dapp MJ, Clouser CL, Patterson S, Mansky LM: 5-Azacytidine can induce lethal mutagenesis in human immunodeficiency virus type 1. J Virol. 2009 Nov;83(22):11950-8. Epub 2009 Sep 2. PMID 19726509
- ^ Knox C, Law V, Jewison T, Liu P, Ly S, Frolkis A, Pon A, Banco K, Mak C, Neveu V, Djoumbou Y, Eisner R, Guo AC, Wishart DS (2011). „DrugBank 3.0: a comprehensive resource for omics research on drugs”. Nucleic Acids Res. 39 (Database issue): D1035—41. PMC 3013709 . PMID 21059682. doi:10.1093/nar/gkq1126.
- ^ David S. Wishart; Craig Knox; An Chi Guo; Dean Cheng; Savita Shrivastava; Dan Tzur; Bijaya Gautam; Murtaza Hassanali (2008). „DrugBank: a knowledgebase for drugs, drug actions and drug targets”. Nucleic acids research. 36 (Database issue): D901—6. PMC 2238889 . PMID 18048412. doi:10.1093/nar/gkm958.
- ^ Ghose, A.K.; Viswanadhan V.N. & Wendoloski, J.J. (1998). „Prediction of Hydrophobic (Lipophilic) Properties of Small Organic Molecules Using Fragment Methods: An Analysis of AlogP and CLogP Methods”. J. Phys. Chem. A. 102: 3762—3772. doi:10.1021/jp980230o.
- ^ Tetko IV, Tanchuk VY, Kasheva TN, Villa AE (2001). „Estimation of Aqueous Solubility of Chemical Compounds Using E-State Indices”. Chem Inf. Comput. Sci. 41: 1488—1493. PMID 11749573. doi:10.1021/ci000392t.
- ^ Ertl P.; Rohde B.; Selzer P. (2000). „Fast calculation of molecular polar surface area as a sum of fragment based contributions and its application to the prediction of drug transport properties”. J. Med. Chem. 43: 3714—3717. PMID 11020286. doi:10.1021/jm000942e.
Literatura
уреди- Hardman JG, Limbird LE, Gilman AG (2001). Goodman & Gilman's The Pharmacological Basis of Therapeutics (10. изд.). New York: McGraw-Hill. ISBN 0071354697. doi:10.1036/0071422803.
- Thomas L. Lemke; David A. Williams, ур. (2007). Foye's Principles of Medicinal Chemistry (6. изд.). Baltimore: Lippincott Willams & Wilkins. ISBN 0781768799.